class: center, middle, inverse, title-slide # A Brief Introduction to Multi-Stage Trials for Developing Dynamic Treatment Regimens ### Nicholas J. Seewald, Ph.D. ### 22 October 2021 Working Group on Clinical Research, University of Rochester Medical Center --- class: inverse, center, middle # Follow along! Slides are available at [urmc-smarts-2021.netlify.app](urmc-smarts-2021.netlify.app) Materials are partially adapted from workshops given by the [d3lab](https://d3lab.isr.umich.edu) at the University of Michigan Institute for Social Research and from <a name=cite-seewaldSequentialMultipleAssignment2021></a>[Seewald, Hackworth, and Almirall (2021)](https://doi.org/10.1007/978-3-319-52677-5_280-1). --- # What is a dynamic treatment regimen? A **dynamic treatment regimen (DTR)** is - an *intervention* design that - adapts the type, timing, intensity, or dose of treatment over time - according to a patient's specific and changing needs. -- In practice, a dynamic treatment regimen is a sequence of decision rules that can be used to guide how treatment can be adapted and readapted to an individual. -- .center[ ### Sounds a lot like clinical practice! ] -- **MANY other names**: adaptive intervention, adaptive treatment strategy, individualized treatment rule, treatment algorithm, individualized intervention... --- class: inverse, center, middle # A dynamic treatment regimen is an *intervention design*, NOT an experimental design. We'll talk about experiments soon! --- # 5 components of a DTR Dynamic treatment regimens consist of 1. Decision points 1. Tailoring variable(s) 1. Intervention options 1. Decision rule(s) 1. Proximal and distal outcomes --- # Example DTR: Alcohol Use Disorder - **Naltrexone** is known to diminish pleasurable effects of alcohol <a name=cite-oslinTargetingTreatmentsAlcohol2006></a>([Oslin, Berrettini, and O'Brien, 2006](https://doi.org/10/fgcfbk)) - Response to naltrexone is heterogeneous (adherence, biology, social support, etc.) <a name=cite-nahum-shaniSMARTDataAnalysis2017></a>([Nahum-Shani, Ertefaie, Lu, Lynch, McKay, Oslin, and Almirall, 2017](https://doi.org/10/ghpb9n)) - Heterogeneity necessitates a supportive intervention alongside naltrexone - **Medical management**: face-to-face clinical support + adherence monitoring - **Combined behavioral intervention**: targets patient's motivation for change, emphasizes social support <a name=cite-longabaughOriginsIssuesOptions2005></a>([Longabaugh, Zweben, Locastro, and Miller, 2005](https://doi.org/10/ghpb9f)) --- # Example DTR: Alcohol Use Disorder  -- 1. Decision points 1. Tailoring variable(s) 1. Intervention options 1. Decision rule(s) 1. Proximal and distal outcomes --- # Example DTR: Decision Points A **decision point** is a time at which treatment might be adapted to the individual.  - **Decision point 1:** Treatment outset -- decide how to initiate treatment - **Decision point 2:** Adjust treatment after 8 weeks --- # Example DTR: Tailoring Variables A **tailoring variable** is used to individualize treatment at each decision point. Can be static (age, baseline risk, etc.) or dynamic (adherence to treatment, disease severity, etc.)  --- # Example DTR: Tailoring Variables .center[] Here, the tailoring variable is the **number of self-reported heavy drinking days following treatment initiation**. - `\(\geq\)` 2 heavy drinking days `\(\rightarrow\)` _non-responder_ - `\(<\)` 2 heavy drinking days `\(\rightarrow\)` _responder_ --- # Some Notes on Tailoring Variables Note that the tailoring variable is _pre-specified_ and _well-defined_. - Tailoring variables **are part of the intervention**! - Should be based on _practical_, _ethical_, or _scientific_ considerations. -- .center[ ### We'll come back to how to choose a tailoring variable. ] --- # Example DTR: Intervention Options - **Intervention options** at each decision point might be aspects of treatment: type, intensity, dose, delivery method, timing, etc. - Here, intervention options are combinations of _naltrexone_, _medical management_, and _combined behavioral intervention_ .center[  ] --- # Example DTR: Intervention Options - In subsequent stages, could be _adaptation strategies_ like "augment", "intensify" or "stay the course". .center[  ] --- # Example DTR: Decision Rules .center[  ] A **decision rule** recommends an _intervention option_ for individuals at each decision point, possibly based on prior information (i.e., a tailoring variable) --- # Example DTR: Decision Rules .center[  ] A **decision rule** recommends an _intervention option_ for individuals at each decision point, possibly based on prior information (i.e., a tailoring variable) --- # Example DTR: Proximal & Distal Outcomes A dynamic treatment regimen's design should be guided by both short-term (**proximal**) and long-term (**distal**) outcomes - **Distal outcomes** are the long-term goals of the DTR - Long-term, example DTR should reduce _likelihood of alcohol use relapse_ - **Proximal outcomes** are the near-term goals of the DTR; perhaps a mechanism by which we can achieve the distal outcome. - Short-term, we want to lower risk of relapse by reducing the _proportion of drinking and heavy-drinking days over 8 weeks after adaptation_ <a name=cite-leiSMARTDesignBuilding2012></a>([Lei, Nahum-Shani, Lynch, Oslin, and Murphy, 2012](https://doi.org/10.1146/annurev-clinpsy-032511-143152)) --- # Scientific Questions about DTRs Often unanswered questions about how to sequence and adapt interventions! Typically related to - relative effectiveness of different DTRs - relative effectiveness of different intervention options - how intervention options work with/against each other -- ###Common Questions 1. Which treatment option should the dynamic treatment regimen begin with? 1. How should we modify treatment for initial non-responders? 1. How should we modify treatment for initial responders? 1. How do we _define_ response/non-response? 1. How should we time decision points? --- # Alcohol Use Disorder: Scientific Questions - How do we define non-response to naltrexone in the context of a dynamic treatment regimen? - Unclear how many heavy drinking days indicate a need to augment treatment - What treatments should be offered following the initial intervention (naltrexone + medical management)? - More intense support for non-responders? - Do responders need a supportive intervention alongside naltrexone? -- ## Let's now discuss an experimental design which can help answer these questions --- class: inverse, middle, center # Sequential, Multiple-Assignment Randomized Trials (SMARTs)  --- # SMARTs A **sequential, multiple-assignment randomized trial** is _one type_ of randomized trial design that can be used to answer questions at multiple stages of the development of a high-quality dynamic treatment regimen. -- The key feature of a SMART is that some (or all) participants are randomized _more than once_. --- # Example: The ExTENd Study (PI: D. Oslin) .center[  ] --- # 8 DTRs "embedded" in ExTENd .center[  ] --- # 8 DTRs "embedded" in ExTENd .center[  ] --- # 8 DTRs "embedded" in ExTENd .center[  ] --- # 8 DTRs "embedded" in ExTENd .center[  ] --- # 8 DTRs "embedded" in ExTENd .center[  ] --- # How do SMARTs inform DTR development? Randomizations in a SMART correspond to open scientific questions about constructing a dynamic treatment regimen! In ExTENd, - First randomization seeks to answer a question about definition of non-response - Second randomization in responders investigates whether lower-intensity medical management ("telehealth") is needed as maintenance therapy - Second randomization in non-responders looks at the effect of naltrexone in the context of combined behavioral intervention + medical management --- # Do I need a SMART? SMARTs are designed to answer questions about the development of high-quality dynamic treatment regimes. Consider a SMART if... 1. You want to develop a DTR 1. There are open questions preventing the construction of an effective DTR 1. There are open questions at _multiple decision points_ within a DTR -- If any of these aren't true, you don't need a SMART! --- # Do I need a SMART? ## What if we know what to do for responders? .center[  ] --- # Do I need a SMART? ## What if we know what to do for responders? .center[  ] --- # Do I need a SMART? ## What if we know what to do initially? .center[  ] --- # Do I need a SMART? ## What if we know what to do initially? .center[ 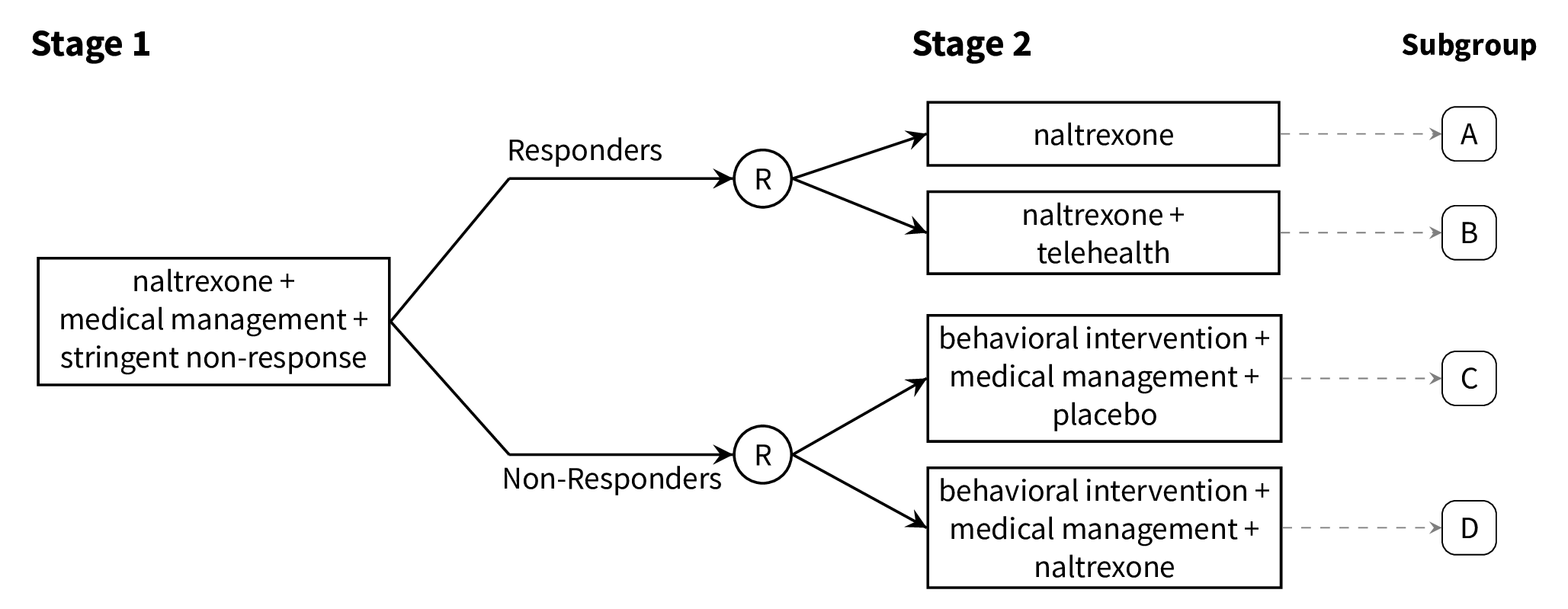 ] --- # Design Considerations: Tailoring Variables Tailoring variables are often used to **restrict randomization** (recommend different intervention options to different subgroups of participants) Tailoring variables should be **well-justified**: remember, they're part of the DTRs! You do **not** need to restrict randomization! --- # Design Considerations: Tailoring Variables If you restrict randomization, do so based on ethical, scientific, and/or practical considerations. -- - **Ethical:** Treatment options should be appropriate for the subset of participants -- - **Scientific:** Options should be based on already-established empirical evidence -- - **Practical:** You might save more intense or costly options for those who need it most (e.g.) -- ### Ultimately, _keep it simple_. --- # Design Considerations: Aims Focus on a few scientific aims about developing a high-quality DTR. - **Primary aim:** Choose sample size based on this aim - **Secondary aims:** Leverage rich data on treatment sequences to further inform a DTR --- # Design Considerations: Primary Aims ## Compare initial intervention options .center[ 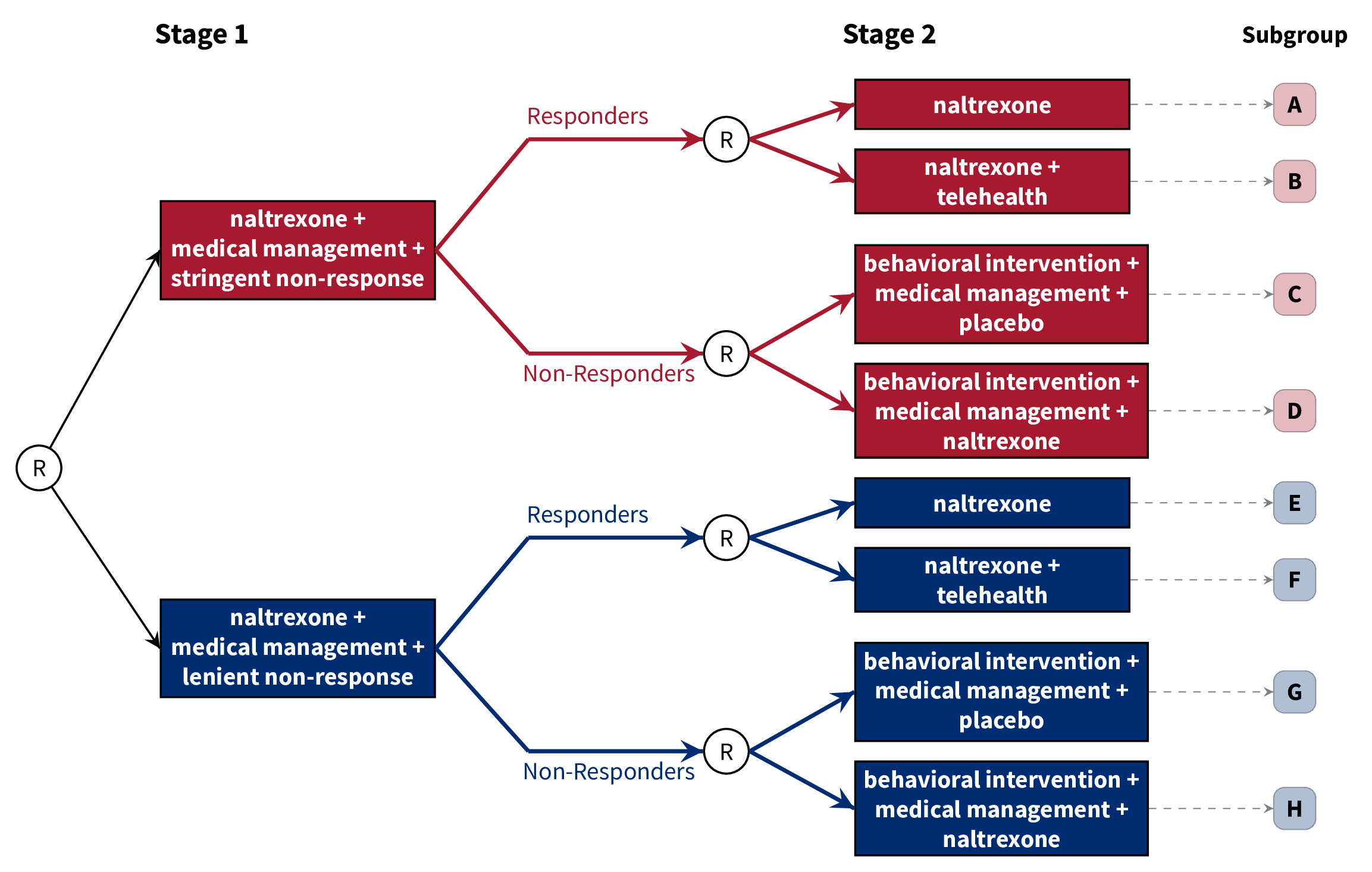 ] --- # Design Considerations: Primary Aims ## Compare initial intervention options .pull-left[ .center[  ] ] .pull-right[ Hypothetical hypothesis might be: > A stringent definition of non-response in the context of a dynamic treatment regimen will lead to fewer heavy drinking days, on average, than a lenient definition of non-response. ] --- # Design Considerations: Primary Aims ## Compare initial intervention options .pull-left[ .center[  ] ] .pull-right[ - Analysis is just a two-group comparison of subgroups A-D vs. subgroups E-H - Sample size requirements are the same as for a two-arm RCT. ] --- # Design Considerations: Primary Aims ### Compare second-stage options among (non-)responders .center[ 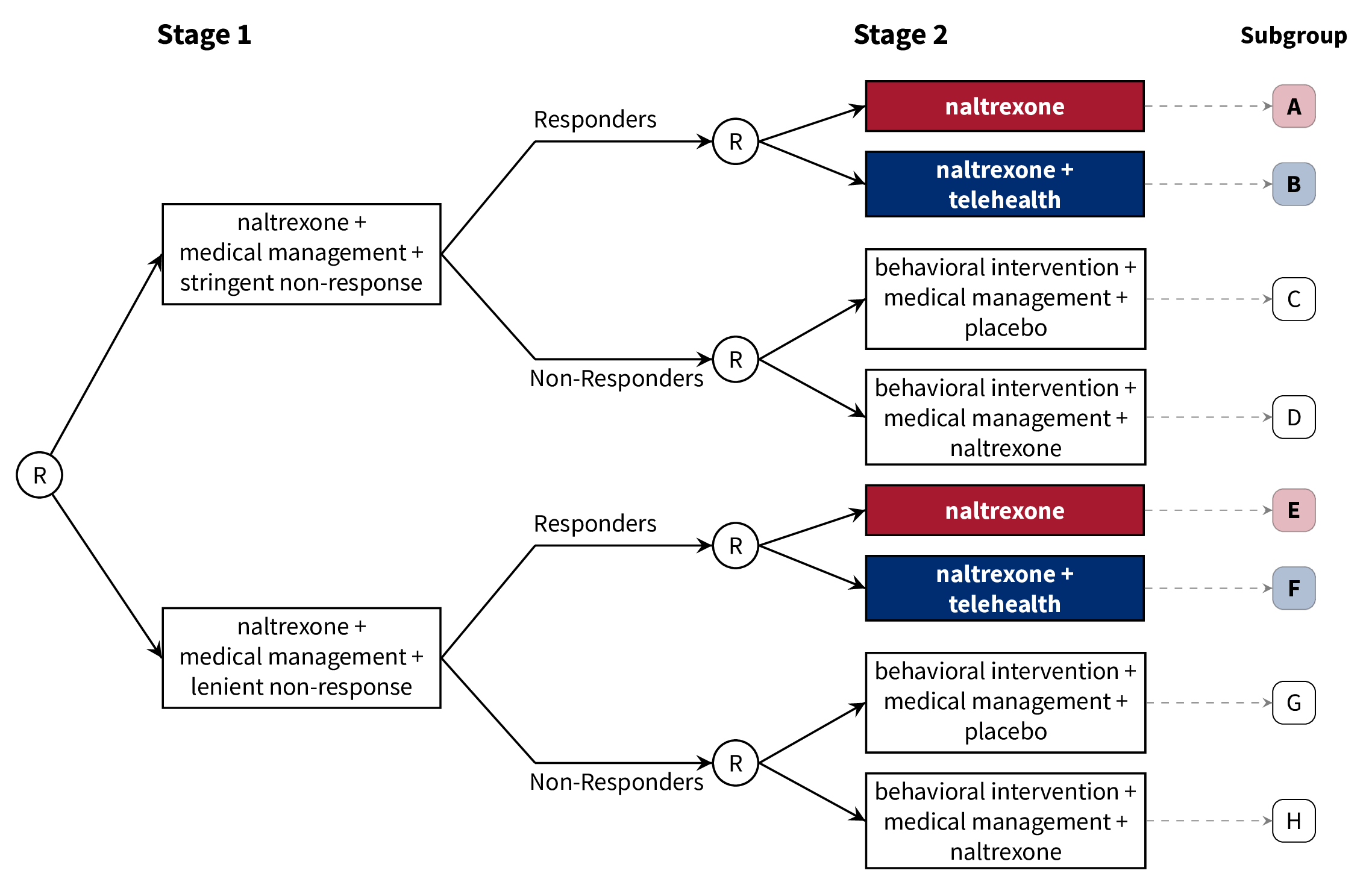 ] --- # Design Considerations: Primary Aims ### Compare second-stage options among (non-)responders .pull-left[ .center[  ] ] .pull-right[ Hypothetical hypothesis: > Individuals who "respond" to naltrexone and medical management benefit more from naltrexone along with a telehealth intervention than naltrexone alone, on average, in terms of proportion of heavy drinking days after 8 weeks. ] --- # Design Considerations: Primary Aimss ## Compare second-stage options among (non-)responders .pull-left[ .center[  ] ] .pull-right[ - Analysis is a two-group comparison of subgroups A & E vs. subgroups B & F - Sample size is as for a two-group comparison, upweighted by response rate. ] --- # Design Considerations: Primary Aims ## Compare embedded dynamic treatment regimens .center[ 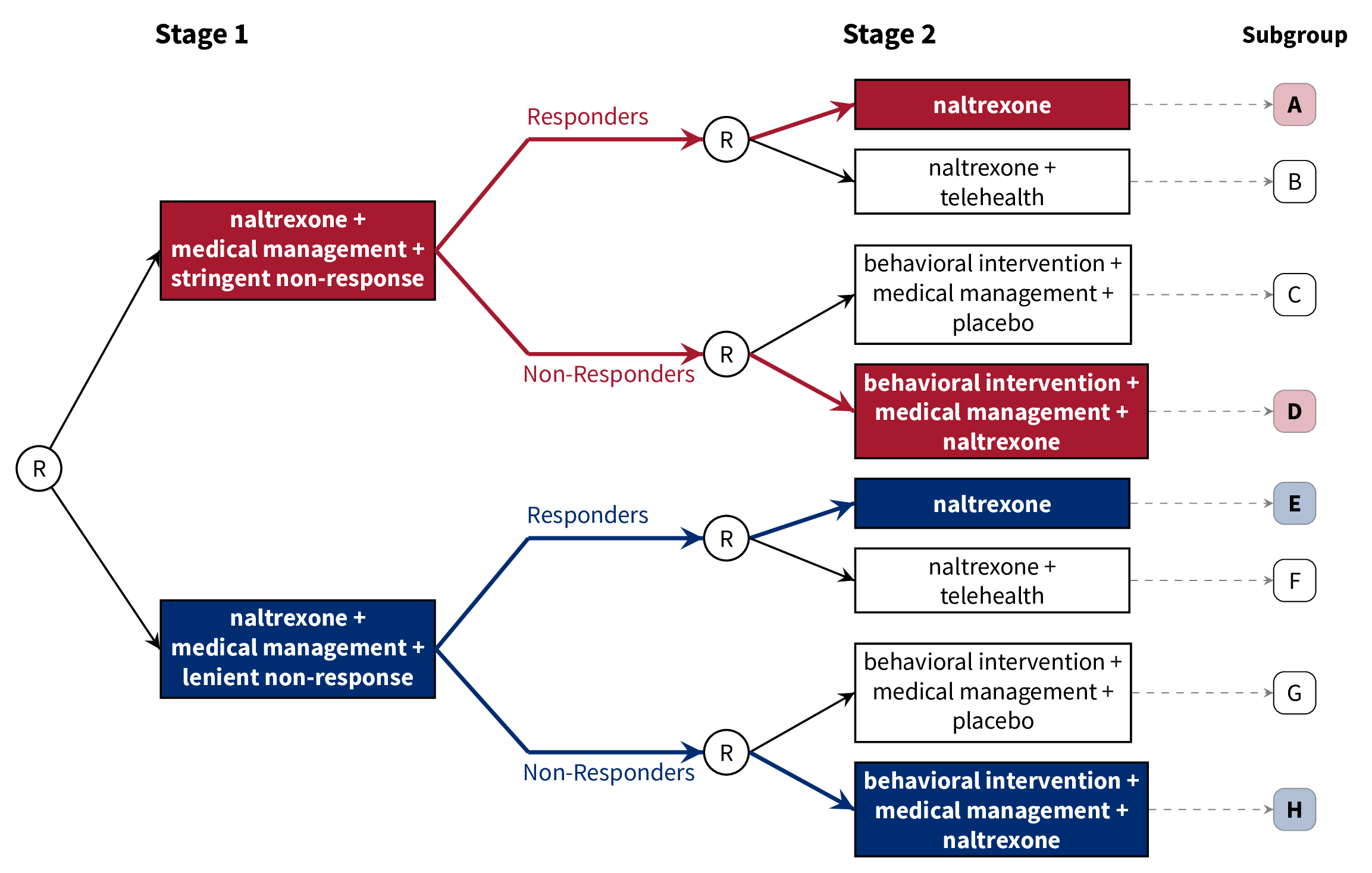 ] --- # Design Considerations: Primary Aims ## Compare embedded dynamic treatment regimens .pull-left[ .center[  ] ] .pull-right[ Hypothetical hypothesis: > The red DTR will lead to fewer heavy drinking days after 8 weeks, on average, as compared to the blue DTR. ] --- # Design Considerations: Primary Aims ## Compare embedded dynamic treatment regimens .pull-left[ .center[  ] ] .pull-right[ - Analysis is *slightly* more complicated, but still - Sample size formulae available (see additional resources) ] --- # Sample Size Resources ## for comparing dynamic treatment regimens - **Continuous Outcomes:** <a name=cite-oettingStatisticalMethodologySMART2011></a><a name=cite-ogbagaberDesignSequentiallyRandomized2016></a>[Oetting, Levy, Weiss, and Murphy (2011)](#bib-oettingStatisticalMethodologySMART2011); [Ogbagaber, Karp, and Wahed (2016)](https://doi.org/10.1002/sim.6747) - **Binary Outcomes:** <a name=cite-kidwellDesignAnalysisConsiderations2018></a>[Kidwell, Seewald, Tran, Kasari, and Almirall (2018)](https://doi.org/10.1080/02664763.2017.1386773) - **Survival Outcomes:** <a name=cite-fengSampleSizeTwostage2009></a><a name=cite-liSampleSizeFormulae2011></a>[Feng and Wahed (2009)](https://doi.org/10.1002/sim.3593); [Li and Murphy (2011)](https://doi.org/10.1093/biomet/asr019) - **Cluster-Randomized:** <a name=cite-necampComparingClusterlevelDynamic2017></a>[NeCamp, Kilbourne, and Almirall (2017)](https://doi.org/10.1177/0962280217708654) - **Longitudinal:** <a name=cite-seewaldSampleSizeConsiderations2020></a>[Seewald, Kidwell, Nahum-Shani, Wu, McKay, and Almirall (2020)](https://doi.org/10/gf85ss) - **Finding the best embedded DTR:** <a name=cite-artmanPowerAnalysisSMART2018></a>[Artman, Nahum-Shani, Wu, Mckay, and Ertefaie (2018)](https://doi.org/10/ggth75) --- # Additional Reading Materials from 3-day workshop on SMARTs and DTRs: <https://d3lab.isr.umich.edu/workshop/getting-smart-about-adaptive-interventions-in-education-2019/> More in-depth, full book on DTRs: Kosorok, M.R., and E.E.M. Moodie, eds. 2015. Adaptive Treatment Strategies in Practice: Planning Trials and Analyzing Data for Personalized Medicine. Philadelphia, PA: Society for Industrial and Applied Mathematics. https://doi.org/10.1137/1.9781611974188. Overview of many different SMART in the field: Lei, H., I. Nahum-Shani, K. Lynch, D. Oslin, and S.A. Murphy. “A ‘SMART’ Design for Building Individualized Treatment Sequences.” Annual Review of Clinical Psychology 8, no. 1 (2012): 21–48. https://doi.org/10.1146/annurev-clinpsy-032511-143152. --- # Additional Reading A nice introduction to SMARTs and DTRs in behavioral science: Nahum-Shani, I., M. Qian, D. Almirall, W.E. Pelham, B. Gnagy, G.A. Fabiano, J.G. Waxmonsky, J. Yu, and S.A. Murphy. “Experimental Design and Primary Data Analysis Methods for Comparing Adaptive Interventions.” Psychological Methods 17, no. 4 (2012): 457–77. https://doi.org/10.1037/a0029372. An introduction to "Q-learning" for building more "deeply tailored" DTRs: Nahum-Shani, I., M. Qian, D. Almirall, W.E. Pelham, B. Gnagy, G.A. Fabiano, J.G. Waxmonsky, J. Yu, and S.A. Murphy. 2012. “Q-Learning: A Data Analysis Method for Constructing Adaptive Interventions.” Psychological Methods 17 (4): 478–94. https://doi.org/10.1037/a0029373. --- # References <a name=bib-artmanPowerAnalysisSMART2018></a>[Artman, W. J. et al.](#cite-artmanPowerAnalysisSMART2018) (2018). "Power Analysis in a SMART Design: Sample Size Estimation for Determining the Best Embedded Dynamic Treatment Regime". In: _Biostatistics_. DOI: [10/ggth75](https://doi.org/10%2Fggth75). <a name=bib-fengSampleSizeTwostage2009></a>[Feng, W. et al.](#cite-fengSampleSizeTwostage2009) (2009). "Sample Size for Two-Stage Studies with Maintenance Therapy". In: _Statistics in Medicine_ 28.15, pp. 2028-2041. ISSN: 0277-6715. DOI: [10.1002/sim.3593](https://doi.org/10.1002%2Fsim.3593). <a name=bib-kidwellDesignAnalysisConsiderations2018></a>[Kidwell, K. M. et al.](#cite-kidwellDesignAnalysisConsiderations2018) (2018). "Design and Analysis Considerations for Comparing Dynamic Treatment Regimens with Binary Outcomes from Sequential Multiple Assignment Randomized Trials". In: _Journal of Applied Statistics_ 45.9, pp. 1628-1651. ISSN: 0266-4763, 1360-0532. DOI: [10.1080/02664763.2017.1386773](https://doi.org/10.1080%2F02664763.2017.1386773). <a name=bib-leiSMARTDesignBuilding2012></a>[Lei, H. et al.](#cite-leiSMARTDesignBuilding2012) (2012). "A "SMART" Design for Building Individualized Treatment Sequences". In: _Annual Review of Clinical Psychology_ 8.1, pp. 21-48. ISSN: 1548-5943, 1548-5951. DOI: [10.1146/annurev-clinpsy-032511-143152](https://doi.org/10.1146%2Fannurev-clinpsy-032511-143152). --- # References <a name=bib-liSampleSizeFormulae2011></a>[Li, Z. et al.](#cite-liSampleSizeFormulae2011) (2011). "Sample Size Formulae for Two-Stage Randomized Trials with Survival Outcomes". In: _Biometrika_ 98.3, pp. 503-518. ISSN: 00063444. DOI: [10.1093/biomet/asr019](https://doi.org/10.1093%2Fbiomet%2Fasr019). <a name=bib-longabaughOriginsIssuesOptions2005></a>[Longabaugh, R. et al.](#cite-longabaughOriginsIssuesOptions2005) (2005). "Origins, Issues and Options in the Development of the Combined Behavioral Intervention." In: _J. Stud. Alcohol Suppl._, pp. 179-187. ISSN: 0363-468X. DOI: [10/ghpb9f](https://doi.org/10%2Fghpb9f). <a name=bib-nahum-shaniSMARTDataAnalysis2017></a>[Nahum-Shani, I. et al.](#cite-nahum-shaniSMARTDataAnalysis2017) (2017). "A SMART Data Analysis Method for Constructing Adaptive Treatment Strategies for Substance Use Disorders". In: _Addiction_ 112.5, pp. 901-909. ISSN: 1360-0443. DOI: [10/ghpb9n](https://doi.org/10%2Fghpb9n). --- # References <a name=bib-necampComparingClusterlevelDynamic2017></a>[NeCamp, T. et al.](#cite-necampComparingClusterlevelDynamic2017) (2017). "Comparing Cluster-Level Dynamic Treatment Regimens Using Sequential, Multiple Assignment, Randomized Trials: Regression Estimation and Sample Size Considerations". In: _Statistical Methods in Medical Research_ 26.4, pp. 1572-1589. ISSN: 0962-2802, 1477-0334. DOI: [10.1177/0962280217708654](https://doi.org/10.1177%2F0962280217708654). <a name=bib-oettingStatisticalMethodologySMART2011></a>[Oetting, A. I. et al.](#cite-oettingStatisticalMethodologySMART2011) (2011). "Statistical Methodology for a SMART Design in the Development of Adaptive Treatment Strategies". In: _Causality and Psychopathology: Finding the Determinants of Disorders and Their Cures_. Ed. by P. E. Shrout, K. M. Keyes and K. Ornstein. New York: Oxford University Press, pp. 179-205. ISBN: 978-0-19-975464-9. <a name=bib-ogbagaberDesignSequentiallyRandomized2016></a>[Ogbagaber, S. B. et al.](#cite-ogbagaberDesignSequentiallyRandomized2016) (2016). "Design of Sequentially Randomized Trials for Testing Adaptive Treatment Strategies". In: _Statistics in Medicine_ 35.6, pp. 840-858. DOI: [10.1002/sim.6747](https://doi.org/10.1002%2Fsim.6747). --- # References <a name=bib-oslinTargetingTreatmentsAlcohol2006></a>[Oslin, D. W. et al.](#cite-oslinTargetingTreatmentsAlcohol2006) (2006). "Targeting Treatments for Alcohol Dependence: The Pharmacogenetics of Naltrexone". In: _Addiction Biology_ 11.3-4, pp. 397-403. ISSN: 1369-1600. DOI: [10/fgcfbk](https://doi.org/10%2Ffgcfbk). <a name=bib-seewaldSequentialMultipleAssignment2021></a>[Seewald, N. J. et al.](#cite-seewaldSequentialMultipleAssignment2021) (2021). "Sequential, Multiple Assignment, Randomized Trials (SMART)". In: _Principles and Practice of Clinical Trials_. Ed. by S. Piantadosi and C. L. Meinert. Cham: Springer International Publishing, pp. 1-19. ISBN: 978-3-319-52677-5. DOI: [10.1007/978-3-319-52677-5_280-1](https://doi.org/10.1007%2F978-3-319-52677-5_280-1). <a name=bib-seewaldSampleSizeConsiderations2020></a>[Seewald, N. J. et al.](#cite-seewaldSampleSizeConsiderations2020) (2020). "Sample Size Considerations for Comparing Dynamic Treatment Regimens in a Sequential Multiple-Assignment Randomized Trial with a Continuous Longitudinal Outcome". In: _Stat Methods Med Res_ 29.7, pp. 1891-1912. ISSN: 0962-2802. DOI: [10/gf85ss](https://doi.org/10%2Fgf85ss). --- class: center, inverse, middle # Reach out with questions (or ideas)! <svg aria-hidden="true" role="img" viewBox="0 0 512 512" style="height:1em;width:1em;vertical-align:-0.125em;margin-left:auto;margin-right:auto;font-size:inherit;fill:currentColor;overflow:visible;position:relative;"><path d="M464 64H48C21.49 64 0 85.49 0 112v288c0 26.51 21.49 48 48 48h416c26.51 0 48-21.49 48-48V112c0-26.51-21.49-48-48-48zm0 48v40.805c-22.422 18.259-58.168 46.651-134.587 106.49-16.841 13.247-50.201 45.072-73.413 44.701-23.208.375-56.579-31.459-73.413-44.701C106.18 199.465 70.425 171.067 48 152.805V112h416zM48 400V214.398c22.914 18.251 55.409 43.862 104.938 82.646 21.857 17.205 60.134 55.186 103.062 54.955 42.717.231 80.509-37.199 103.053-54.947 49.528-38.783 82.032-64.401 104.947-82.653V400H48z"/></svg> [nseewal1@jhu.edu](mailto:nseewal1@jhu.edu) <svg aria-hidden="true" role="img" viewBox="0 0 512 512" style="height:1em;width:1em;vertical-align:-0.125em;margin-left:auto;margin-right:auto;font-size:inherit;fill:currentColor;overflow:visible;position:relative;"><path d="M459.37 151.716c.325 4.548.325 9.097.325 13.645 0 138.72-105.583 298.558-298.558 298.558-59.452 0-114.68-17.219-161.137-47.106 8.447.974 16.568 1.299 25.34 1.299 49.055 0 94.213-16.568 130.274-44.832-46.132-.975-84.792-31.188-98.112-72.772 6.498.974 12.995 1.624 19.818 1.624 9.421 0 18.843-1.3 27.614-3.573-48.081-9.747-84.143-51.98-84.143-102.985v-1.299c13.969 7.797 30.214 12.67 47.431 13.319-28.264-18.843-46.781-51.005-46.781-87.391 0-19.492 5.197-37.36 14.294-52.954 51.655 63.675 129.3 105.258 216.365 109.807-1.624-7.797-2.599-15.918-2.599-24.04 0-57.828 46.782-104.934 104.934-104.934 30.213 0 57.502 12.67 76.67 33.137 23.715-4.548 46.456-13.32 66.599-25.34-7.798 24.366-24.366 44.833-46.132 57.827 21.117-2.273 41.584-8.122 60.426-16.243-14.292 20.791-32.161 39.308-52.628 54.253z"/></svg> [@nickseewald](https://twitter.com/nickseewald) <svg aria-hidden="true" role="img" viewBox="0 0 512 512" style="height:1em;width:1em;vertical-align:-0.125em;margin-left:auto;margin-right:auto;font-size:inherit;fill:currentColor;overflow:visible;position:relative;"><path d="M326.612 185.391c59.747 59.809 58.927 155.698.36 214.59-.11.12-.24.25-.36.37l-67.2 67.2c-59.27 59.27-155.699 59.262-214.96 0-59.27-59.26-59.27-155.7 0-214.96l37.106-37.106c9.84-9.84 26.786-3.3 27.294 10.606.648 17.722 3.826 35.527 9.69 52.721 1.986 5.822.567 12.262-3.783 16.612l-13.087 13.087c-28.026 28.026-28.905 73.66-1.155 101.96 28.024 28.579 74.086 28.749 102.325.51l67.2-67.19c28.191-28.191 28.073-73.757 0-101.83-3.701-3.694-7.429-6.564-10.341-8.569a16.037 16.037 0 0 1-6.947-12.606c-.396-10.567 3.348-21.456 11.698-29.806l21.054-21.055c5.521-5.521 14.182-6.199 20.584-1.731a152.482 152.482 0 0 1 20.522 17.197zM467.547 44.449c-59.261-59.262-155.69-59.27-214.96 0l-67.2 67.2c-.12.12-.25.25-.36.37-58.566 58.892-59.387 154.781.36 214.59a152.454 152.454 0 0 0 20.521 17.196c6.402 4.468 15.064 3.789 20.584-1.731l21.054-21.055c8.35-8.35 12.094-19.239 11.698-29.806a16.037 16.037 0 0 0-6.947-12.606c-2.912-2.005-6.64-4.875-10.341-8.569-28.073-28.073-28.191-73.639 0-101.83l67.2-67.19c28.239-28.239 74.3-28.069 102.325.51 27.75 28.3 26.872 73.934-1.155 101.96l-13.087 13.087c-4.35 4.35-5.769 10.79-3.783 16.612 5.864 17.194 9.042 34.999 9.69 52.721.509 13.906 17.454 20.446 27.294 10.606l37.106-37.106c59.271-59.259 59.271-155.699.001-214.959z"/></svg> [nickseewald.com](https://www.nickseewald.com)